Introduction
We already know what a battery is. We come across it almost every day and it has become an integral part of our lives. For example, the smartphone that you use. It runs on a compact form of lithium-ion battery. But just having a battery is not enough. We require a good battery which can run our appliance for a justified period of time. You will become frustrated if you have to charge your smartphone battery after every few hours of use. So you will look for a battery that takes a minimal amount of time for charging and gives out an output for say at least a day after one full cycle of charge. The same theory applies to IOT devices that run on batteries. An IOT device is nothing but a simple device that has been connected to the internet for the purpose of exchanging information. Every IOT device has different power requirements. A simple sensor node will be able to operate on an AA battery or even a coin cell with an output voltage of 3V and will require very less current. But an IOT device that needs to run a motor will need something more than that. It will require a battery that can give up to 2 Amperes of current, like a sealed lead acid battery with an output voltage of 12V.
Types of batteries
Most of the batteries that we use are referred to as chemical batteries in general. The function of a chemical battery is simple, convert chemical energy into electrical energy and vice versa. Battery types depend on a lot of criteria. For example, some applications require short bursts of power whereas some require a continuous supply. Some require high current to run while some will work fine with low current values. From the application point of view, batteries differ and have to be selected wisely, especially in iot field where battery life is of paramount importance. There are a lot of options to choose from, some of which are given below:
- Lithium: Lithium batteries come in a lot of varieties. Most famous ones used in IoT applications are in button or coin format
- Button type (BR) lithium cells are made from lithium alloy and carbon mono-fluoride gel. This cell type has a voltage of 3V and drops till 2.2V when discharged. It has a low self-discharge rate and is ideal for devices that are meant to run for longer duration and have low power requirements.They are usually used in RTC and memory backups. Common models include BR2032 ( 190 mah ) , BR1225 ( 48 mah ) etc .

Image taken from source
- Coin type (CR) lithium cells have higher discharge rate compared to button type. They are used in devices that are not meant to be run for long duration but require higher pulse currents. The CR type cells use Manganese Dioxide as the cathode which reduces the internal impedance of the battery.They are used where pulse current is required like remotes , small wireless devices , flashes . Common models include CR2032 ( 225 mah ) , CR2025 ( 165 mah ) etc.

Image taken from source
- Zinc-air: These cells have an excellent energy density but also high self-discharge rate. Because of this, they are usable for only a few months and are not recommended.

Image taken from source
- Alkaline: These are the most popular batteries that we have been seeing for a long time. It is used in low duty cycle applications mostly. The nominal voltage that a cell possesses is 1.5V and drops up to 0.9V.

Image taken from source
- Zinc-Carbon: These batteries tend to have a low self-discharge rate similar to alkaline type and can be used up to a period of ten years. But the energy density is very less which leads to poor performance. These batteries are used only when low cost is under consideration.

Image taken from source
- Silver-Oxide: These are based on same chemistry as alkaline and are made from, as the name suggests, silver oxide cathode. The self-discharge rate is very less. It has the ability to provide high power levels without affecting overall capacity. The energy density of these cells is also very high compared to Alkaline type. The only drawback is that these cells are very costly because of the material used in them.

Image taken from source
- Lithium Thionyl Chloride: These cells are comparatively newer and have a lot of plus points. The nominal voltage is 3.6V and after discharging reaches 2.2V. The self-discharge rate is extremely low as claimed by the manufacturer.

Image taken from source
- Nickel Cadmium(NiCd): This battery has two electrodes which are made of nickel oxide hydroxide and metallic cadmium. This is a rechargeable battery with the nominal cell voltage of 1.2V and cycle durability of 2,000 cycles. With a relatively low internal resistance, they can supply high surge currents which makes them an ideal choice for remote-controlled toys, electrical models, and camera flash units. These batteries suffer from something called memory effect, that is, if they are discharged and recharged up to the same level of charge a few times, they produce a voltage drop at the particular level. Because of this, the batteries appear dead earlier than normal. Specific energy ranges from 40 to 60Wh/kg. Specific Power is 150W/kg. The life cycle is 2000 cycles.

Image taken from source
- Nickel-Metal Hydride(NIMH): This is a rechargeable battery. In this battery, the positive electrode uses nickel oxide hydroxide (NiOOH) and the negative electrode uses a hydrogen absorbing alloy. A NiMH battery has much more capacity compared to an equivalent NiCd battery (up to 2 to 3 times). This battery has a nominal voltage of 1.2V and cycle durability ranging from 180 - 2000 cycles. Charging voltage is in range of 1.4V - 1.6V per cell. A discharged cell gives out around 1.25V/cell during discharge and goes up to 1V/cell. Going below that level will damage the battery permanently. This battery is commonly available in AA format. This battery finds its application in electric plug-in vehicles. Specific energy ranges from 60 to 120 Wh/kg. Specific power ranges from 250 to 1000 W/kg. The life cycle is 180 to 2000 cycles.

Image taken from source
- Lead Acid: This is a rechargeable battery. It has low energy to weight ratio and low energy to volume ratio. This battery has the ability to supply high surge currents. It has a nominal cell voltage of 2.1V and cycles durability of fewer than 350 cycles. The self-discharge rate for this battery ranges from 3% to 20% in one month. Due to its low cost, it finds its application in a lot of areas. The most common application is in automobile ignition. Specific energy is 33 to 42 Wh/kg. Specific power is 180 W/kg.

Image taken from source
- Lithium Ion: Lithium Ion battery contains a negative and a positive electrode between which the lithium ions flow. During discharge, they move from negative to the positive electrode and during charging they move from positive to the negative electrode. They have an intercalated lithium compound that serves as an electrolyte. These compounds consist of lithium salts in an organic solvent such as ethylene carbonate. Specific energy ranges from 100 to 265 Wh/kg. Specific power ranges from 250 to 340 W/kg. Life cycle ranges from 400 to 1200 cycles.

Image taken from source
- Lithium-Ion Polymer: Lithium Ion Polymer batteries are the most common type of batteries that we come across from day to day life. They are also referred to as LiPo, LIP etc. These are rechargeable batteries which use High conductivity semi-solid or gel like polymer electrolyte. These batteries are implemented in cases where weight and size are crucial. These batteries have an energy density of 0.90-2.63 MJ/L. The voltage of LiPO depends on the chemistry of the battery. To know the discharged voltage and charging voltage, product’s datasheet must be consulted. The problem with these batteries is that it is affected by overcharge,over-discharge,over-temperature, short circuit etc which causes it to either bloat, leak or catch fire.

Image taken from source
- Lithium Cobalt: This is a type of Lithium-ion battery that has Cobalt as the main active material. It has a high specific energy (150–200Wh/kg). It consists of a cobalt oxide cathode and a graphite carbon anode. During discharge, Lithium ions move from anode to cathode which has a layered structure. The drawback of this type of battery is that it has a comparatively shorter lifespan and less thermal stability. Also, the specific power is limited. Nominal voltage is 3.6V. Cycle life ranges from 500 to 1000 cycles. The cost of this battery is higher as the cost of Cobalt is high.

Image taken from source
- Lithium Manganese: This battery has high thermal stability and low internal cell resistance that enables fast charging and high current discharging. The overall performance of this battery is moderate as the capacity is lower than cobalt variant. The nominal voltage is 3.7V to 3.8V. Specific energy capacity is in the range of 100 to 150 Wh/kg. The life cycle varies from 300 to 700 cycles. This battery is safer than the Cobalt version. This battery is mixed with other active metals to enhance a particular trait like specific energy (capacity of the battery), specific power (load capability and longevity.

Image taken from source
- Lithium Phosphate: This battery has a low nominal voltage 3.2V/cell. Also, the specific energy is lesser than the cobalt variant. Cold temperature reduces the performance. This battery has a higher self-discharge rate. Specific energy ranges from 90 to 120 Wh/kg. The life cycle is in between 1000 to 2000 cycles. This battery has very flat voltage discharge curve. It has a low capacity. From the safety perspective, this is one of the safest Lithium Ion batteries.

Image taken from source
- Lithium Titanate: In this battery, the graphite anode is interchanged with Lithium Titanate. The nominal cell voltage of Lithium Titanate is 2.4V and has the ability to discharge high current while maintaining the capacity(which is also high). This battery can also be fast charged. It can perform well at lower temperatures, unlike other Lithium-ion batteries. Specific energy is in between 50 to 80 Wh/kg. The life cycle is in the range 3000 to 7000. The drawback of this kind of battery is that it is expensive. But it is also among the safest Lithium Ion batteries.

Image taken from source
Comparison table
|
Battery Type
|
Anode(-) |
Cathode(+) |
Nominal Voltage (V) |
Approx. Energy Density (MJ/kg) |
Characteristics
|
|
Alkaline

|
Zinc |
Manganese dioxide |
1.5 |
0.5 |
+ High Current Capacity
+ High Current Drain
+ Low Discharge ( 3% / year)
- More expensive compared to Zinc Carbon
|
|
Zinc-Carbon

|
Zinc |
Manganese dioxide |
1.5 |
0.13 |
+Economical , Low cost batteries
- Low Current Drain
- Low Temperature Operating Range ( -5 to 55 C)
- High Self Discharge ( 30 % / year )
- Possibility of Leakage |
|
Lithium (BR)

|
Lithium |
Carbon monofluoride |
3 |
1.3 |
+Higher temperature range compared to CR series
+high internal impedance
+Stable voltage and current discharge curve
+Low Self Discharge ( .5% / year )
-low pulse current
|
|
Lithium(CR)

|
Lithium |
Manganese dioxide |
3 |
1 |
+Good pulse current capability
+Stable voltage during discharge
+Low self Discharge ( 1% / year )
+Low Cost due to wide usage
- Tapered Discharge profile
|
|
Lithium-thionyl chloride

|
Lithium |
Sulfur-oxygen chlorine |
3.6 |
1.04 |
+Wide Operating Temperature ( -55 to 85 C )
+ Very Low self Discharge ( .08 % / month )
+ High Current Capcity
- Low Current Discharge Rate
- Hazardous Electrolyte and cathode
|
|
Zinc-air

|
Zinc |
Oxygen |
1.4 |
1.69 |
High energy density, short battery life(weeks or months)
|
|
Nickel Cadmium

|
Cadmium |
Nickel Hydroxide |
1.2 |
50–150 W·h/L |
Fast, simple charge, a higher number of charge-discharge cycles, relatively low energy density, suffers from the memory effect
- High Self Discharge ( 15 % / month )
|
|
Nickel-Metal Hydride

|
Hydrogen storing metal alloy |
Nickel hydroxide |
1.2 |
140–300 Wh/L |
Higher storing capacity, lesser memory effect problem, environment-friendly, limited discharge current
- High Self Discharge ( 30% / month )
|
|
Lead Acid

|
Porous lead |
Lead dioxide |
2.1 |
60–110 Wh/L |
Least expensive, reliable, low maintenance, high discharge rate capability, low energy density, not environment-friendly
- Medium Self Discharge (5% / month)
|
|
Lithium-Ion

|
Graphite |
Metal Oxide |
3.6 |
250–693 W·h/L |
High energy density, relatively low self-discharge, low maintenance, requires voltage and current protection circuit, aging effect
- Medium Self Discharge ( 3% / month)
|
|
Lithium-Ion Polymer

|
Graphite |
Metal Oxide |
3.6 |
100–265 W·h/kg |
Flexible form factor, lightweight, lower energy density, less life cycle
|
|
Lithium Cobalt

|
Graphite |
Lithium cobalt oxide |
3.6 |
150–200Wh/kg |
Expensive, high specific energy density, limited specific power
|
|
Lithium Manganese

|
Graphite |
Lithium Manganese Oxide |
3.7 |
100–150Wh/kg |
Less capacity, high power, used in medical devices and electric powertrains
|
|
Lithium Phosphate

|
Graphite |
Lithium iron phosphate |
3.3 |
90–120Wh/kg |
Good voltage discharge capability, less capacity, relatively high self-discharge, used in applications demanding high load current
|
| Lithium Titanate

|
Graphite |
Lithium titanate |
2.4 |
50–80Wh/kg |
Good temperature compatible range, longer life compared to other batteries, faster rate of charging, low specific energy, expensive
|
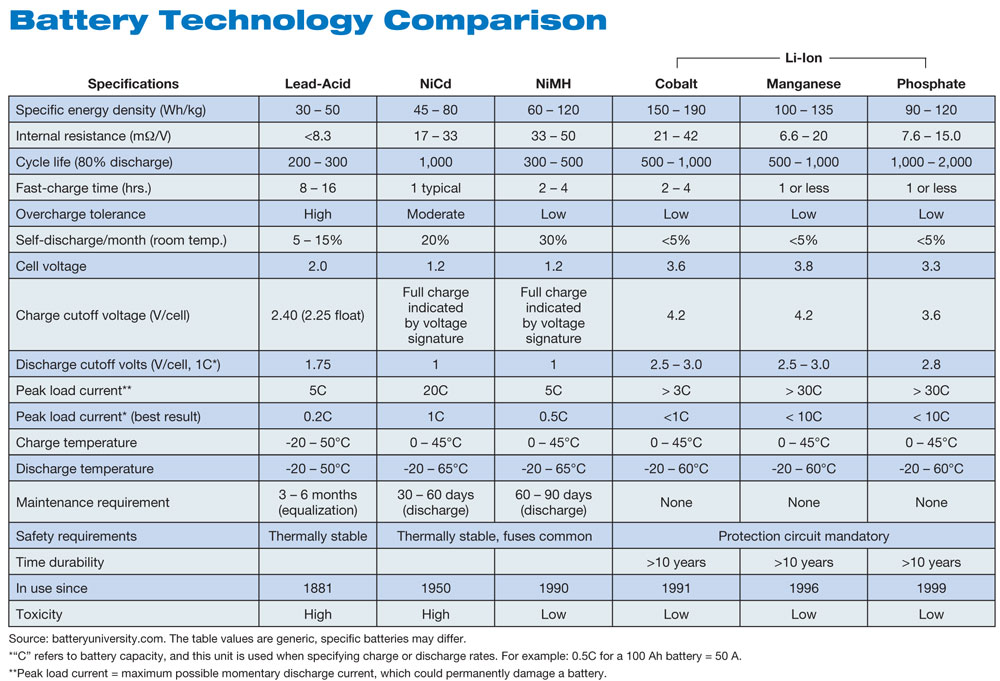
Image taken from source
Basic cost idea for IoT batteries
|
Battery Type
|
Cost $/Watt hr
|
|
Lead Acid
|
$0.17
|
|
Alkaline
|
$0.19
|
|
Zinc Carbon
|
$0.31
|
|
NiMH
|
$0.99
|
|
NiCad
|
$1.50
|
|
Lithium-ion
|
$0.47
|
Factors to be considered before making a choice
Following factors need to be taken into consideration before deciding which battery to use:
- The nominal voltage at which the device can operate.
- Total operating duration of the device.
- The number of times the battery is discharged and recharged.
- Amount of time required for charging.
- The cut-off voltage of the battery.
- Physical dimensions of the device that needs to incorporate the battery.
- Environmental and electromagnetic characteristics that will affect the life of battery directly or indirectly.
- Overall cost
> Nominal voltage is the least voltage at which your device will operate. The battery that you choose must have a minimum voltage rating that either is equal to the device’s nominal voltage or is lesser than that.
> Operating duration of the device gives an idea about the battery capacity. Battery capacity is measured in Ah (Ampere-Hour) or Wh (Watt-Hour). Another common unit is mAh. A milliampere hour is 1000th of an ampere-hour (Ah).
> The number of times the battery can be recharged, retention of charge and response to trickle charge has to be taken into consideration to decide the life of the battery.
> Cut off voltage is important in applications where you want to implement a feature that lets you know the end of battery charge. Knowing the cut off voltage, you can easily implement a circuit that disconnects the battery when it has reached that value.
> Physical characteristics of the battery are also important. For example, a wearable IoT device will need a really small and thin battery, but on a larger scale like smart home automation, battery size consideration will be irrelevant.
> Environmental considerations to consider are battery's ability to reject moisture, corrosion, overheating, bloating, withstand shock and damage. For example, if the IOT device has to be installed in the field, the battery used must have a good waterproofing system in it.
> The final factor is cost. If the overall cost of the IOT device has to be kept low, then battery cost plays a prominent role.


